 In a recent Boston Globe article, reporter Jenn Abelson covered a $1.5 million jury verdict in favor of a Malden man, Carlos Osorio, in a products liability action against a table saw manufacturer that claimed the saw was defective because it was not equipped with “flesh detection technology” that would have caused the blade to stop when it got too close to human flesh. Yes, such “flesh detection technology” does exist and is quite effective.
In a recent Boston Globe article, reporter Jenn Abelson covered a $1.5 million jury verdict in favor of a Malden man, Carlos Osorio, in a products liability action against a table saw manufacturer that claimed the saw was defective because it was not equipped with “flesh detection technology” that would have caused the blade to stop when it got too close to human flesh. Yes, such “flesh detection technology” does exist and is quite effective.
Flesh detection technology is just another example of a safety device that would probably not exist if there were no products liability lawyers to force manufacturers to internalize the costs that society incurs through the use of dangerous and defective products. Table saws without such safety technology are cheaper. Most table saws are probably purchased and used by construction companies, who generally can’t be sued by their employees because of Worker’s Compensation technology. Do you think construction companies would be willing to shell out extra for a premium model? It seems unlikely. But manufacturers sell saws equipped with flesh detection technology because it’s cheaper to offer safety technology than to pay up in lawsuits.
A couple generations ago, products liability lawyers were the ones who got manufacturers to adopt safety guards and other “bells and whistles.”
If you see a lawyer today, give her a hug. She may have saved your fingers from being sawed off.
Category Archives: Dangerous and Defective Products
Plaintiff Loses In First Seroquel Trial To Go To Jury Verdict
It’s been reported that the first Seroquel trial to go to jury verdict anywhere in the country has resulted in a loss for the plaintiff, with jurors voting 7-1 on Wednesday that AstraZeneca, Seroquel’s manufacturer, adequately warned the plaintiff’s doctors about Seroquel’s putting the plaintiff at an elevated risk for diabetes.
Seroquel is a pharmaceutical most often used to treat patients for bipolar disorder, schizophrenia or other mental illnesses, but is frequently also prescribed as a sedative and as a treatment for restless leg syndrome. Seroquel is AstraZeneca’s second-biggest selling drug.
A number of people prescribed Seroquel have filed lawsuits against AstraZeneca, claiming that the drug maker failed to warn adequately that the drug can lead to diabetes and pancreatitis.
The Seroquel lawsuits filed in federal court have all been consolidated into a single class action, however, individual lawsuits have proceeded in various state courts. This Seroquel defense action was one such case; it was filed on behalf of a single plaintiff in New Jersey.
It should be noted that verdict does not mean that the jury found that Seroquel does not cause diabetes. What the jury was simply supposed to consider was the question of whether AstraZeneca, Seroquel’s makers, adequately warned the patient’s doctors of the risks in the materials they provided the doctors.
In October of 2009, AstraZeneca settled two whistleblower lawsuits about Seroquel for $520 million and agreed to enter into a “Corporate Integrity” agreement with the United States Department of Justice. The allegations raised by the company’s internal whistleblower concern clinical trials of Seroquel but are otherwise confidential. Should the details of these cases also become public, we will report on that. In the meantime, we will keep you posted as these Seroquel cases reach jury verdict in other states.
FDA To Investigate Link Between Osteoporosis Drugs And Femur Fractures
In response to research and media reports suggesting a link between a certain class of osteoporosis drugs – known as oral bisphosphonates – and femur fractures, the FDA announced yesterday that it is working with outside experts to insure that the osteoporosis drugs are safe.
Fosamax, Actonel, Boniva and Reclast are all oral bisphosphonates, the class of drugs under investigation. Some have claimed that long-term use of these drugs raises the risk of an unusual type of femur fracture just below the hip that is known as a subtrochantreric fracture.
The Wall Street Journal reports that two studies that were presented this week at the American Academy of Orthopaedic Surgeons’ annual meeting suggests that long-term use of Fosamax and Boniva (where “long-term” means four or more years of use) may lead to reduced “bone structural integrity.”
In 2008, the FDA warned that Fosamax had been lined to serious joint pain.
The FDA cautioned yesterday, however, that people taking oral bisphosphonates should not stop taking the drugs without first consulting with their physicians. At this point, the FDA believes that the benefits of Fosamax and the other oral bisphosphonates outweigh the potential risks that it is investigating.
The Tort Reform Crowd Thinks This Was A Frivolous Lawsuit
Reading the tort reform blog Pointoflaw.com, I came across a link captioned: “‘Ford failed to warn seating unsafe for obese persons’ suit fails.” Sounds pretty frivolous, right?
I followed the link to Abnormal Use, a corporate defense blog, which has the virtue of being intellectually honest, unlike Pointoflaw. There I got the whole story.
It wasn’t just some overweight person who was suing Ford for failure to warn a chair might collapse under her weight.
The plaintiff in the case was a 300 pound woman driving a Ford Explorer that was rear-ended by another SUV at the (relatively low) speed of 30 mph. The impact of the accident caused her seat to collapse backwards. The accident also left her a paraplegic.
Ford hadn’t tested or designed the seats for anyone above 220 pounds. Now here’s a little graph that I just found through a simple google search that shows about 5-10 percent of men are in the 220 pound range. Yet Ford didn’t bother testing above 220 pounds.
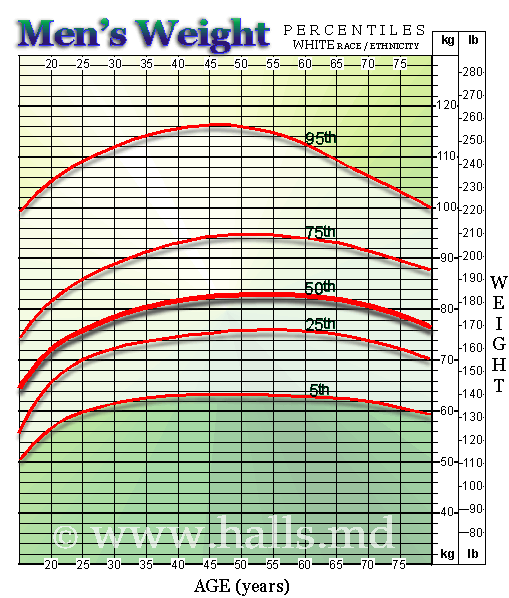
And this poor woman wound up a paraplegic in a 30 mph accident. Sound frivolous now?
Yet, according to the blog post, the trial court entered a directed verdict against her on her failure-to-warn claim and didn’t even let that claim get to the jury.
I assumed that this woman’s failure-to-warn claim was not her only claim and that she also brought breach of warranty claims against Ford. I was hoping that she managed to prevail on one of those other claims. I went to the (unreported) decision on Westlaw. It appears the other claim did make it to jury and that the jury found against her.
I have a great respect for juries and jury verdicts so I’ll leave alone the fact that the jury found against her on a strict liability standard that should’ve been unfavorable to Ford.
But the online reporting about this case illustrates how the media stir up worries about frivolous lawsuits.
This wasn’t an overweight person who sued Ford because the seat collapsed under her weight and she fell on her butt. This was a woman who was rendered a paraplegic, in part, because Ford didn’t test its seats above a weight range within which some ten percent of the adult male population falls. And she wasn’t suing Ford for failing to include a warning label on the chair saying, “If you’re too heavy, this seat may cause injuries.” Her failure-to-warn claim was just one of the legal theories she pursued (her claim for breach of the warranty of merchantability was much stronger).
From a societal perspective, cases like these should really boil down to: Who should bear the cost? As a paraplegic, this woman will require millions of dollars in medical care for the rest of her life. Who should bear the cost of that? We, the taxpayers, or Ford, a company that profits from a car seat that it never bothered to test at 30 mph with a dummy weighing more than 220 pounds? If Ford has to bear the cost do you think that maybe next time they might design a better seat?
Good NPR Program On Toyota Recall
NPR’s OnPoint program, broadcast from Boston’s own WBUR, had a terrific hour-long program on the Toyota recall today. You can listen here.
FDA Announces Effort To Reduce Radiation Overdose Medical Malpractice
In my January 25 blog post, I discussed a phenomenon that should be of concern to patients in Massachusetts and elsewhere – the prevalence of medical malpractice involving radiation overdose.
This week, the Food and Drug Administration announced its “Initiative to Reduce Unnecessary Radiation Exposure from Medical Imaging.” The initiative will focus on two goals: 1.) insuring the medical necessity of any imaging procedure and 2.) optimizing the amount of radiation necessary for each procedure.
Over the past two decades, Americans’ exposure to ionizing radiation has nearly doubled. This increase in radiation exposure is largely attributable to exposure from CT scans, fluoroscopy and nuclear medicine. According to one study, the CT scans performed in the United States in 2007 alone could lead to 29,000 additional cases of cancer down the road.
It will be interesting to see how successful the FDA is in persuading the medical profession to use medical imaging only when necessary, especially in light of how profitable such procedures are for hospitals.
The corporate defense blog Mass Tort Defense has more on this story.
Toyota Recall II: Is Sudden Uncontrolled Acceleration Something To Be Worried About?
The pro-business blog Point of Law insists, citing to an article in Popular Mechanics, that the dangers posed by Toyota’s acceleration system are overblown. Meanwhile, Department of Transportation head Ray LaHood is telling Toyota owners to stop driving (at least until political concerns cause him to backtrack).
Who do you trust?
Someone should tell Popular Mechanics that forty-one percent of reports of sudden uncontrolled acceleration are about Toyotas. Meanwhile Toyota held about 16 percent of US market share in 2009. Sounds statistically significant to me.
Consumer Product Safety Commission Tells Massachusetts Parents To Stop Using Cribs
Today the Consumer Product Safety Commission issued an immediate recall of “Generation 2 Worldwide” and “childESIGNS” drop side cribs because of the risk of death from suffocation or strangulation created by the cribs’ drop side design. The recall notice urges parents to stop using the cribs immediately and not to attempt to fix the cribs’ design flaws. Here is a copy of the notice in its entirety:
Generation 2 Worldwide and “ChildESIGNS” Drop Side Crib Brands Recalled; Three Infant Deaths Reported
WASHINGTON, D.C. – The U.S. Consumer Product Safety Commission (CPSC) is announcing the recall of all Generation 2 Worldwide and “ChildESIGNS” drop side cribs. CPSC is warning parents and caregivers who own these drop side cribs that infants and toddlers are at risk of serious injury or death due to strangulation and suffocation hazards presented by the cribs. CPSC staff urges parents and caregivers to stop using these cribs immediately and find an alternative, safe sleeping environment for their baby. Do not attempt to fix these cribs.
The crib’s plastic hardware can break which can cause the drop side of the crib to detach from a corner of the crib. When the drop side detaches, it creates a space into which an infant or toddler can roll and become wedged or entrapped. When a child is entrapped between the drop side and the crib mattress, it creates a risk of suffocation or strangulation. In addition, the crib’s mattress support can detach from the crib frame, creating a hazardous space in which an infant or toddler could become entrapped and suffocate or strangle.
CPSC has received reports of three infants who suffocated when they became entrapped between the crib mattress and the drop side when the drop side detached. In July 2007, an eight month old child from Newark, Ohio suffocated when he became entrapped between the drop side and the crib mattress. The drop side of his crib had detached due to a broken plastic stop tab on the lower track. In October 2003, an eight month old child from Richmond, Ind. suffocated when he became entrapped between the drop side and the crib mattress. The plastic hardware on the drop side was broken and allowed the drop side to detach from the crib headboard in one corner. In September 2002, a six month old from Staunton, Va. suffocated when he became entrapped between the drop side and crib mattress. The lower drop side track was missing two screws which allowed it to pull away from the headboard post and detach.
CPSC has also received reports of 20 other drop side incidents, 12 of which involved the drop side detaching in a corner of the crib. In two of these incidents, a child became entrapped. One child suffered bruising from the entrapment. There are five reports of children falling out of the cribs due to drop side detachment. One child suffered a broken arm as a result of the fall.
In addition, CPSC has received 8 reports of mattress support detachment in these cribs. Due to the space created by the detachment, three children became entrapped between the crib frame and the sagging mattress and four children crawled out of the crib. There was one report of cuts and bruises.
Due to the fact that Generation 2 went out of business in 2005, CPSC has limited information about the cribs. Although CPSC does not know the total number of units distributed or the years of production, it is believed that there were more than 500,000 of these cribs sold to consumers. Some of the known model numbers are: 10-110X, 10-210X, 21-110X, 20-710X, 64-315X, 26-110X, 90-257X, 20-810X, 46-715X, 64-311X, 74-315X, 21-815X, 21-810X, 20815X, 308154 and 54915. (The “X” denotes where an additional and varying number may appear at the end of the model number.) However, all Generation 2 Worldwide and “ChildESIGNS” drop side cribs are included in this recall, including those with other model numbers.
The name “Generation 2 Worldwide” appears on a label affixed to the crib’s headboard or footboard. Some labels identify the place of manufacture as Dothan, Ala. Others identify China as the country of manufacture. The name “ChildESIGNS” appears on the teething rail of some of the cribs.
The recalled cribs were sold at numerous local furniture and retail stores including Buy Buy Baby, and Kmart and Walmart stores nationwide for between $60 and $160. Consumers should contact the store from which they purchased the crib for remedy information, which will vary between a refund, replacement crib or store credit, depending on the retailer. Consumers are urged to contact CPSC and report any difficulties in obtaining a remedy from their place of purchase.
Important Message from CPSC:
CPSC would like to remind parents not to use any crib with missing, broken, or loose parts. Make sure to tighten hardware from time to time to keep the crib sturdy. When using a drop-side crib, parents should check to make sure the drop-side or any other moving part operates smoothly. Always check all sides and corners of the crib for disengagement. Any disengagement can create a gap and entrap a child. In addition, do not try to repair any side of the crib, especially with tape, wire or rope.
For more information on Crib Safety, visit CPSC’s Crib Information Center.
Picture of Recalled Drop Side Crib Picture of name ‘ChildESIGNS’ on teething rail
Massachusetts Women Should Be Aware Of The Risks of Yaz
According to this news article, fifty Indiana women have sued Bayer, the maker of the Yasmin (or “Yaz”) birth control pill, because their use of the pill caused serious health problems, such as stroke and heart attacks.
Yasmin or “Yaz,” has been sold since 2001. It contains a hormone known as drospirenone that can lead to high levels of blood potassium. High levels of potassium can, in turn, lead to a condition known as hyperkalemia, which can be responsible for heart attacks, blood clots and circulatory problems.
In the past few months, dozens of lawsuits have been filed against Bayer, claiming that Yaz is unsafe. The Food and Drug Administration has issued a warning letter concerning Yaz, however Bayer has not recalled the product.
To date, no Yaz lawsuits have been filed in Massachusetts. We will update you if Massachusetts women do become part of this litigation.
Toyota Acceleration Problem Poses Dangers For Massachusetts Drivers
Earlier this week, a third wrongful death lawsuit was filed against Toyota relating to acceleration problems that have caused Toyota vehicles to accelerate suddenly and uncontrollably.
This third lawsuit was filed by lawyers for Trina Renee Harris, a 34-year old mother of two, who was killed when her 2009 Toyota Corolla slammed into a cement divider on a toll road. There were no skid marks or other evidence of an attempt to brake.
Harris’ apparent inability to stop the car is consistent with reports of other recent Toyota crashes. In August, an off-duty California state trooper and three of his family were killed after the Lexus they were driving accelerated to 120 m.p.h. In a telephone call to 911 that the family made while trapped in the speeding Lexus, the family explained to 911 dispatch that the car was accelerating without their being able to control it.
Another Toyota driver, Bulent Ezal, had his Camry suddenly accelerate in a restaurant parking lot and plunge 70 feet off a cliff, landing in the ocean. Ezal’s wife was killed in this accident.
Thankfully, not all of the accidents have been fatal. One driver, Joseph Hauter, survived a crash that occurred when his 2008 Toyota Camry suddenly accelerated at a gas station. Investigators are looking into several other non-fatal accidents in multiple states.
Thus far, there have not been any reports of Massachusetts Toyota drivers being involved in sudden acceleration crashes. However, Massachusetts drivers need to take precautions because Toyota cars seem especially prone to this problem. As the Consumerist blog reports, 41 percent of sudden acceleration complaints that were made in 2008 were for Toyota and Lexus models.
Lawyers for the car accident victims in these cases believe that the problem lies in an electronic throttle system that was installed in many Toyota models. The electronic throttle system does not have any mechanical link between the accelerator pedal and the engine. In addition, there is no override system for the electronic throttle, so that pressing the brake when the throttle is stuck will not cause the accelerator to shut off.
Toyota has instituted a nationwide recall to attempt to address the problem. The vehicles affected by the recall include:
- 2009-2010 RAV4
- 2009-2010 Corolla
- 2007-2010 Camry
- 2009-2010 Matrix
- 2005-2010 Avalon
- 2010 Highlander
- 2007-2010 Tundra
- 2008-2010 Sequoia
Lexus models were not included in this recall, although, as noted above, Lexuses have been the subject of complaints and at least one wrongful death suit. If you own a Toyota model listed in the recall, or one not listed that you are concerned about, you can call Toyota’s customer service department at 1-800-331-4331.
